 Image via Wikipedia
Image via Wikipedia
The Italian biotechnology company, MolMed S.p.A, announced the expansion of its anticancer drug NGR-hTNF for the treatment of malignant pleural mesothelioma into the United States, Canada and the European Union.
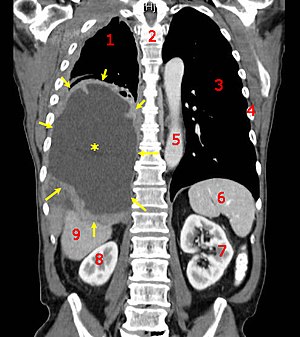
Mesothelioma is a rare cancer linked to asbestos exposure that attacks the lining of the organs – lung, heart and abdomen. The most common is malignant pleural mesothelioma or cancer of the lining of the lungs. Mesothelioma has a long latency period and symptoms are non-specific, so in most cases diagnosis is difficult before the advanced stages of the disease. Typical mesothelioma treatment includes chemotherapy, surgery and radiation in an effort to extend the patient’s life.
MolMed’s NGR-hTNF is an anti-tumor therapy developed for malignant mesothelioma. In more detail, it is a vascular targeting agent with unique mode of action with a first-in-class compound in the class of peptide/cytokine complexes able to selectively target tumor vasculature. NGR-hTNF contains a tumor homing peptide (NGR) that selectively binds tumor blood vessels attached to the human cytokine TNF. Clinical development of NGR-hTNF is ongoing as the sole treatment as well as combined with other treatments of mesothelioma.
Malignant mesothelioma treatment using conventional therapies has not proven to be successful often leaving the affected patients with a poor prognosis and limited survival time. Clinical trials for mesothelioma may offer the best available treatment but participants should be fully aware of the risks. The clinical trial is one of the final stages of the cancer research process and is important in developing new treatments for cancers.
Source: “MolMed Expands Phase III Trial of NGR-hTNF for the Treatment of Mesothelioma….”, February 28, 2011
 Asbestos & Mesothelioma Law Blog
Asbestos & Mesothelioma Law Blog